From Concept to Product Support
Frontier Science lends its expertise to the design, conduct, analysis, and reporting of clinical trials and observational studies. Our knowledgeable team is ready to customize solutions to meet your research needs.
Gather Requirements
With our decades of experience in building solutions, we know how to ask the right questions. We work closely with project stakeholders and domain experts to define the problem to be solved, determine the goal for a project, and identify the right tools to meet that goal.
Design the Solution
To help our partners visualize solutions, we will create mockups of applications, reports, and data to confirm requirements. Using feedback, we will quickly refine designs to ensure that the solution we build meets the project’s needs.
Develop the Solution
Following an iterative model, we work on building applications quickly and reviewing implementations with stakeholders. We use feedback to improve our solution and continue to work with domain experts until all requirements are fully implemented.
Test & Validate
Many of our partners need applications that adhere to strict compliance requirements. Our robust testing and validation process ensures that our work will meet or exceed even the most stringent regulatory needs.
Deploy & Maintain
We do not see our role as ending once a solution is in production. We continue to provide support for users and implement new features needed as your project grows.
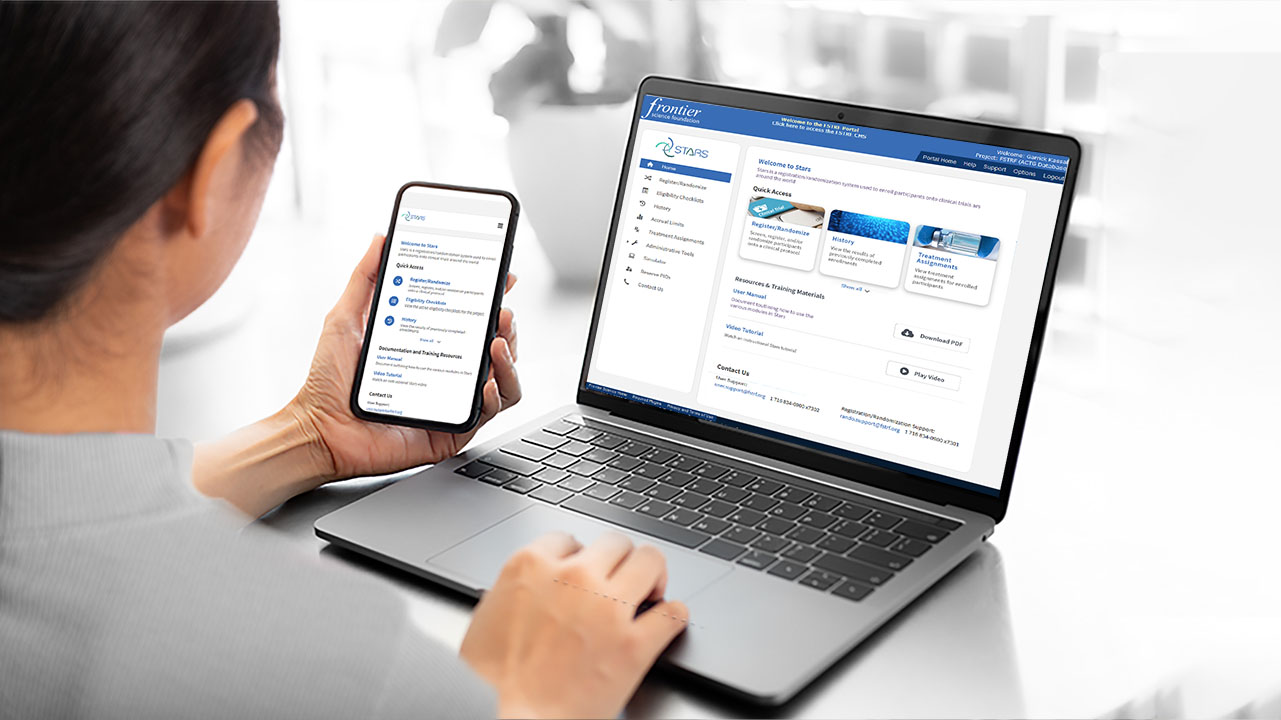
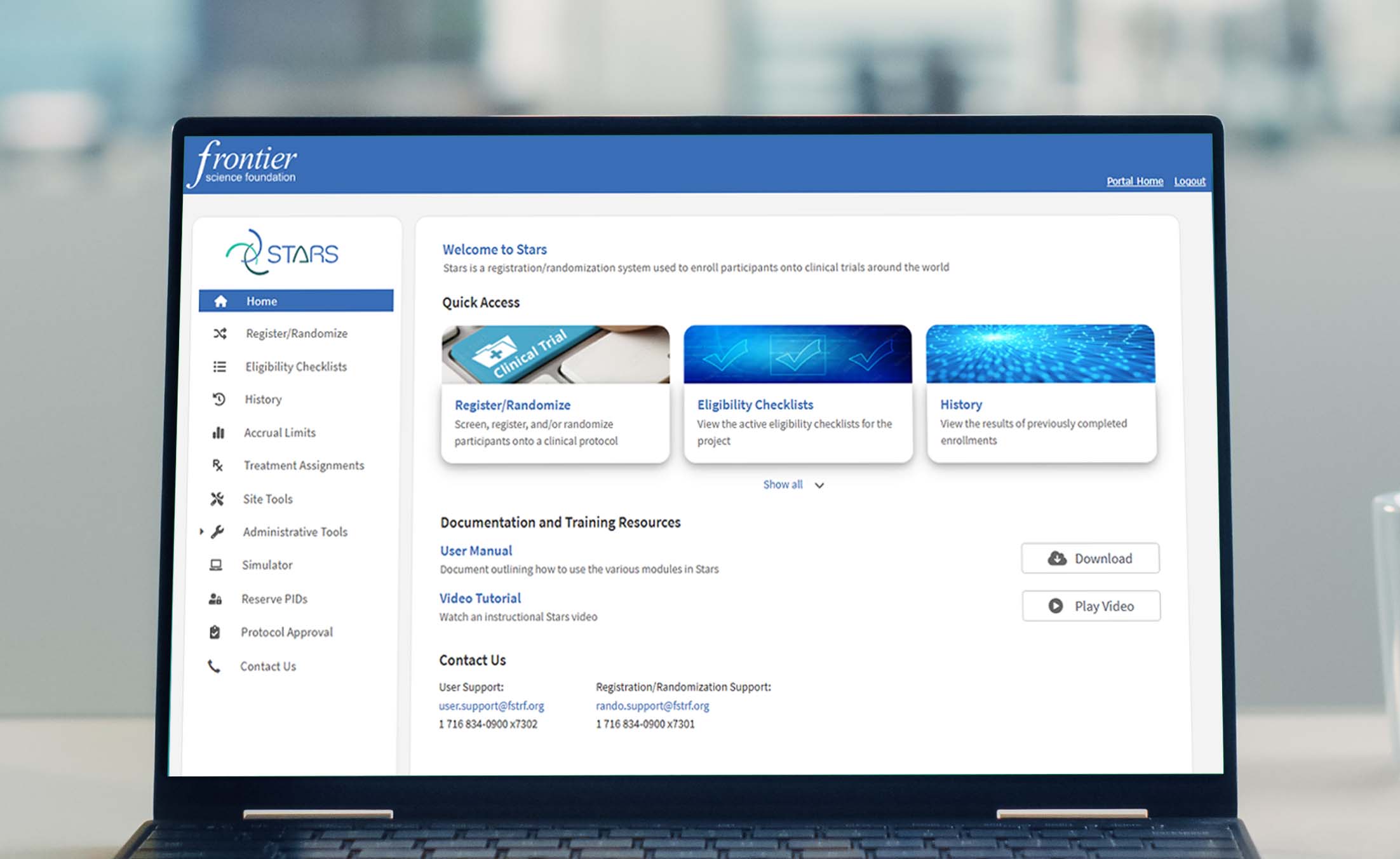
Enrollment and Randomization System
Frontier Science has decades of experience building and administering participant enrollment, registration, and randomization systems. We have built Stars, our solution for enrollment used by numerous large-scale NIH-supported trial networks.
Our in-house support team is available to help study teams and users ensure accurate and reliable registration.
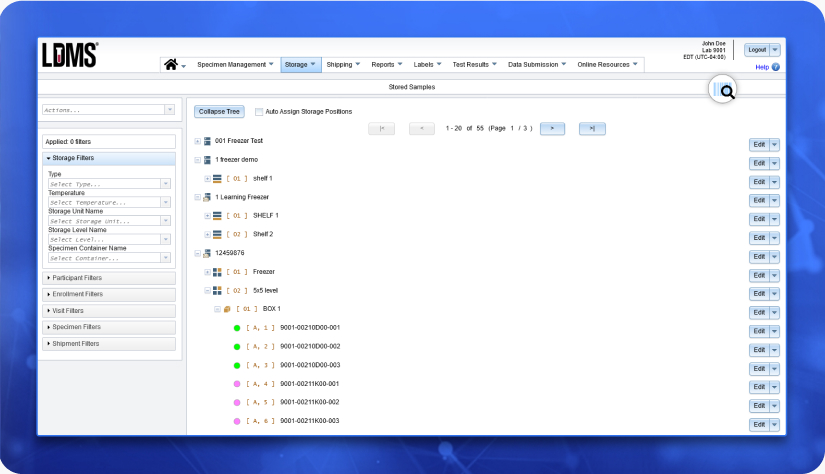
LDMS
LDMS is our comprehensive laboratory information management system for managing collections of biological specimens. It is used by clinics and laboratories around the world to manage specimen tracking, inventory storage, specimen shipment, barcode labeling and much more.
Website Development
Utilizing our reliable development infrastructure and process, we build project websites for many of our partners. These are important assets for promoting the critical research being performed and making resources available to researchers.

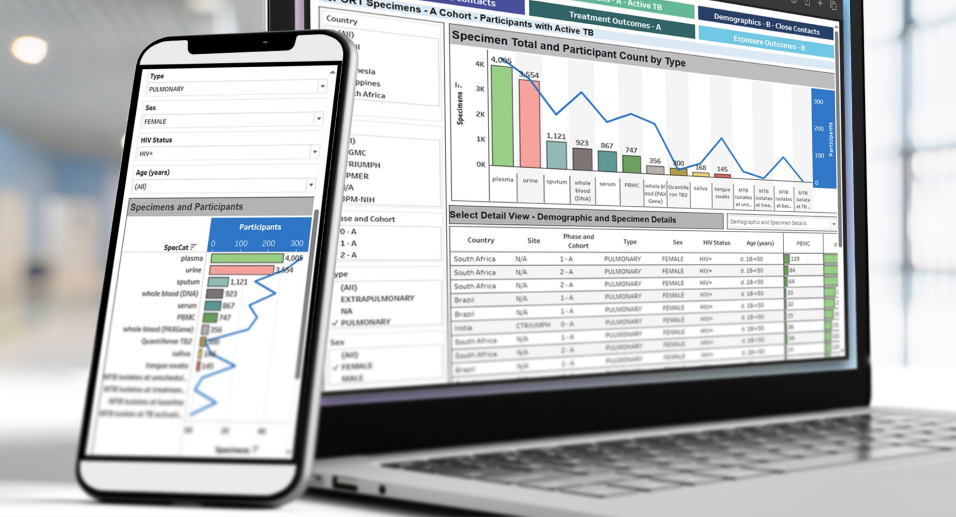
Reports and Visualizations
As data management experts, we transform raw data into actionable insights with on-demand reports and web-based, interactive data dashboards.

Data Integration and Harmonization
Leveraging our data management and transformation expertise, we help partners map and combine raw data to build robust, performant, and accurate data stores.
Compliance and Audits
Partner with us for expertise in regulatory compliance, including 21 CFR Part 11 and GDPR. We provide validation deliverables to boost confidence in your data’s reliability and audit readiness.
Trusted Tools
Our skilled development team bring your ideas to life, creating practical tools for study management. We specialize in comprehensive clinical research solutions including:
Study Enrollment
Electronic Data Capture
Auditing Tools
Our Software Team
Our team of skilled IT professionals, software engineers, web designers, study builders, enrollment support, and database experts works together to develop comprehensive solutions tailored to your needs.

We take great care in designing our infrastructure to adhere to industry standards that meet all relevant hardware and software regulations and software development lifecycle best practices.